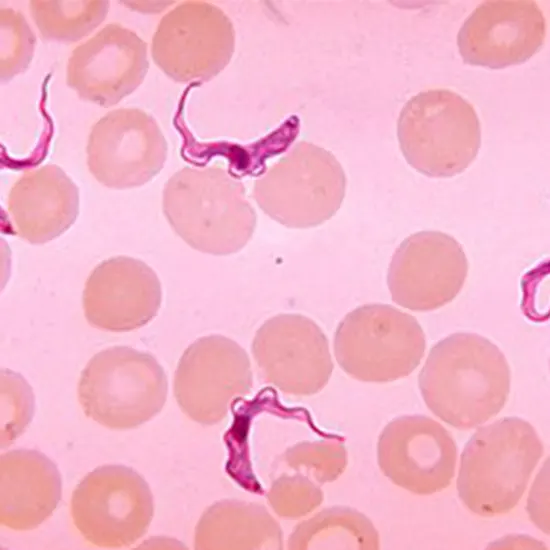
The causes of human African trypanosomiasis, Trypanosoma brucei rhodesiense and T. brucei gambiense, are transmitted by tsetse flies. The parasite undergoes changes inside the vector that get it ready to infect the human host.
The causes of human African trypanosomiasis, Trypanosoma brucei rhodesiense and T. brucei gambiense, are transmitted by tsetse flies. The parasite undergoes changes inside the vector that get it ready to infect the human host. The replicative pro-cyclic (in which the parasite surface is covered by procyclins) and trypo-epimastigote forms, as well as the non-replicative, infectious, metacyclic form that develops in the vector salivary glands, are these developmental stages in chronological order. Metacyclic parasites start to express and become heavily covered in the Variant Surface Glycoprotein (VSG) as a pre-adaptation to their life in humans.
The human host, on the other hand, encourages the parasite's removal by inducing innate and adaptive immune responses and increasing the release of cytokines and chemokine. Overall, the host-parasite relationship is quite dynamic and results in reactions that require several control sites to properly develop.
Trypanosomes provoke a host response and challenge the immune system similarly to other bacteria and parasites. This parasite-host interaction can result in either a weak immune response, which can lead to a devastating hyper-infection, or an excessive immune response that poses a serious threat to life and has equally terrible effects. The parasite must balance its behaviour between these two extremes in order to succeed, avoiding the indiscriminate killing of the host while also avoiding the immune system's destruction. The ability of trypanosomes to successfully evade the effects of immune system activation may be due to the time limitations and periods they shared with humans during their evolution over many million years.
Microbiological diversity and hosts variety
The majority of trypanosome species can only infect animals, mostly by their natural insect vector, the tsetse fly, and are incapable of infecting humans. Human infections are typically prevented by the trypanolytic protein apolipoprotein-L1, two protein complexes called TLF (TLF1 and TLF2), and innate resistance.
However, the species that cause HAT are T. brucei gambiense and T. brucei rhodesiense, which are members of the T. brucei (b.) group (subgenus Trypanozoon). The function of TLF1 and TLF2 factors is overcome and neutralised by processes present in both species. The serum resistance associated gene, which is a shortened VSG, gives resistance to lysis in T. b. rhodesiense. This function is carried out by a more advanced mechanism in T. b. gambiense. It entails a particular shortened VSG (TgsGP), decreased TLF receptor binding affinity, and enhanced cysteine protease activity.
HAT is spread by female tsetse flies (genus Glossing spp.; Steverding) that feed on blood. Pro-cyclic parasites multiply within the vector, and the parasite undergoes changes before entering the metacyclic stage, which is the form that is infectious to the human host. The tsetse fly may inject its saliva into a mammalian host to inoculate the metacyclic form that forms in the salivary glands of the vector .
Blood transfusions are rarely used to transmit HAT . Humans with the condition experience infertility and miscarriage; as a result, placental transfer is not assured. However, the HAT guidelines and procedures emphasise that pregnant women and infants of infected mothers should undergo routine HAT testing .
In reality, T. evansi or T. vivax infections cause thousands of animals to perish each year in Africa, Asia, and South America . Under normal circumstances, T. equiperdum infects horses; by venereal transmission, it may also result in dourine, an illness that affects horses . The slender forms of T. b. brucei, T. b. rhodesiense, and T. b. gambiense are morphologically similar to T. evansi (monomorphic) and T. equiperdum. There is little knowledge available on how these veterinary parasite infections affect the host.As a result, the focus of the current review is primarily on the trypanosomes that infect humans and induce HAT.
Diagnosis of African trypanosomiasis includes microscopic examination of blood, aspiration of lymph nodes, or cs fluid to know about the presence of Trypanosoma brucei parasites. The tests such as Peripheral Blood Smear, Cerebrospinal Fluid Examination Routine and CBC are performed for confirmation.
The imaging techniques such as EEG, MRI Brain with contrast may also be used for confirmation.
Parasite-human host interaction
Role of parasite and host genetics
The parasite's genetic diversity affects how the disease develops. This depends on the particular parasite that is responsible for it. Actually, pathogenesis differs between strains, at least in part, and may include various host pathways. For instance, T. b. gambiense causes the more chronic infection, and T. b. rhodesiense causes the acute HAT sickness .The results suggest that the expression of trypan-tolerance or the existence of trypan-tolerant hosts (mammals that remain infected but do not display the full-blown disease) are the result of the functioning of specific genetic loci. The majority of information currently available related to the role of parasite genetics in the outcome of the disease has been described in experimental infections made in animals.
In fact, despite evidence of asymptomatic carriers and spontaneous cure, the notion of trypano-tolerance in humans has been questioned.In HAT caused by the gambiense strain, for instance—which is thought to be usually fatal—cases of self-cure in untreated patients have been reported, albeit precise diagnostic techniques weren't yet available.High levels of IL-10 and low levels of TNF- have been linked to an increased risk of developing HAT in studies conducted in mangrove areas of coastal Guinea, but high levels of IL-8 were linked to disease prevention.
Tools for Parasites to Modulate Human African Trypanosomiasis Host Response
Sensing the quorum The parasitemia is increased by slender form replication in infected hosts' blood. Trypanosomes regulate their concentrations inside mammalian hosts, so this does not continue indefinitely. Throughout the course of the infection, there is always a balance because, at least in the early stages of the illness, cell growth should be sufficient to ensure infection transmission without outpacing the equilibrium and causing host destruction. The immune system may be able to remove the initial infecting parasites, but as we previously learned, a small percentage of the trypanosome population switches coats, allowing some parasites with a different antigenic specificity on their surface to evade the immune system and survive in the bloodstream long enough to successfully transmit to new hosts.
Pleomorphic and monomorphic trypanosomes both secrete SIF, which is a soluble, low-molecular-weight, heat-stable factor. This is a chemical that accumulates in the culture medium or bloodstream with rising parasite density and is thought to be segregated by thin form parasites. It is suggested that it works as an autocrine trigger molecule that is thin to stumpy in appearance.SIF's identity and mode of action are still not fully understood, however its effector systems appear to contain cAMP or cAMP hydrolysis products. However, contentious recent findings employing the chemical inducer (8-cPT-cAMP/AMP) disqualified the application of the canonical cAMP pathway. Several protein families may be involved in the regulation of stumpy development, albeit the majority of the data need to be confirmed .
Summary
One tool that trypanosomes use to control their hosts is antigenic variation. The major antigenic molecule that may be found on the surface of these blood parasites is the trypanosome VSG. The complex mechanisms by which antigen flipping takes place are probably a factor in the chronicity of infection. The parasite uses coat renewal through VSG-switch to successfully evade the immune system, escape the effects of blood-borne antibodies, and continue to infect the human host.
Because of this, the immune system is only partially successful, and the parasite stays in the bloodstream long enough to spread to a new host—either a human or a blood-sucking insect—via blood-blood contact. The creation of cell-cycle paused parasite transmission stages, which is density-dependent, limits the infection in addition to antigen switching.















