Major prion protein (PrP), is encoded within the human by the PRNP quality moreover known as CD230 (cluster of separation 230). Expression of the protein is most transcendent within the apprehensive framework but happens in...
Major prion protein (PrP), is encoded within the human by the PRNP quality moreover known as CD230 (cluster of separation 230). Expression of the protein is most transcendent within the apprehensive framework but happens in numerous other tissues all through the body.
Structure of PRNP
PrP is profoundly moderated through warm-blooded creatures, loaning assurance to the application of conclusions from test creatures such as mice. Comparison between primates is particularly comparative, extending from 92.9-99.6% similitude in amino corrosive arrangements.
The human protein structure comprises a globular space with three α-helices and a two-strand antiparallel β-sheet, an NH2-terminal tail, and a brief COOH-terminal tail.
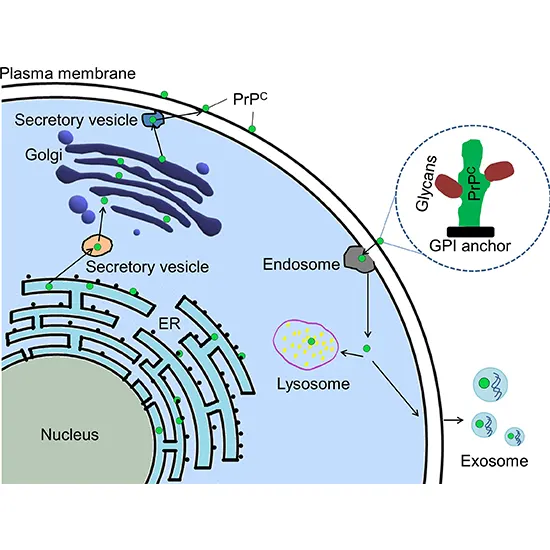
A glycophosphatidylinositol (GPI) film grapple at the COOH-terminal ties PrP to cell layers, and this demonstrates serum vitamin b12 to be indispensable to the transmission of conformational alter; discharged PrP missing the grapple component is unaffected by the irresistible isoform.
The essential grouping of PrP is 253 amino acids long sometime recently post-translational alteration. Flag groupings within the amino- and carboxy-terminal closes are expelled posttranslationally, coming about in a developed length of 208 amino acids.
For human and brilliant hamster PrP, two glycosylated locales exist on helices 2 and 3 at Asn181 and Asn197. Murine PrP has glycosylation destinations such as Asn180 and Asn196. A disulfide bond exists between Cys179 of the moment helix and Cys214 of the third helix (human PrPC numbering).
PrP delivery person RNA contains a pseudoknot structure (prion pseudoknot), which is thought to be included in the control of PrP protein interpretation.
Ligand-binding
The instrument for conformational change to the scrapie isoform is hypothesized to be a slippery ligand-protein, but, so distant, no such compound has been recognized. In any case, a huge body of inquiry has been created on candidates and their interaction with the PrPC.
Copper, zinc, manganese, and nickel are affirmed PrP ligands that tie to its octarepeat region. Ligand official causes a conformational alter with obscure impact. An overwhelming metal official at PrP has been connected to resistance to oxidative stretch emerging from overwhelming metal harmfulness.
PrPC (normal cellular) isoform
Even though the exact work of PrP isn't however known, it is possibly included in the transport of ionic copper to cells from the encompassing environment.
Analysts have moreover proposed roles for PrP in cell signaling or within the arrangement of neural connections. PrPC connects to the external mri brain + dwi surface of the cell film by a glycosylphosphatidylinositol grapple at its C-terminal Ser231.
Prion protein contains five octapeptide rehashes with grouping PHGGGWGQ (though the primary rehash has the slightly-modified, histidine-deficient arrangement PQGGGGWGQ).
Typically thought to generate a copper-binding space through nitrogen particles within the histidine imidazole side-chains and deprotonated amide nitrogens from the 2nd and 3rd glycines within the rehash.
The capacity to tie copper is, in this manner, pH-dependent. NMR appears copper officially comes about in a conformational alter at the N-terminus.
PrPSc (scrapie) isoform
PrPSc may be a conformational isoform of PrPC, but this introduction tends to construct up">to build up in compact, protease-resistant totals inside neural tissue.
The irregular PrPSc isoform incorporates a diverse auxiliary and tertiary structure from PrPC, but indistinguishable essential arrangement. Circular dichroism appears that typical PrPC has 42% alpha-helical and 3ta sheet substance, though PrPSc is as it were 30% alpha helix and 43ta sheet.
However, the nearness of alpha helices in irresistible PrPSc has come into address, with current models proposing a need for alpha helices inside and out, supplanted instep with a total beta sheet composition. This refolding renders the PrPSc isoform extremely resistant to proteolysis.
The proliferation of PrPSc could be a subject of awesome intrigue, as its collection may be a pathological cause of neurodegeneration. Based on the dynamic nature of spongiform encephalopathies, stool routine the overwhelming speculation sets that the alteration from ordinary PrPC is caused by the nearness and interaction with PrPSc.
Strong back for typically taken from considers in which PRNP-knockout mice are safe to the presentation of PrPSc.Despite the far-reaching acknowledgment of the adaptation transformation theory, a few ponder relief claims for a coordinated connection between PrPSc and cytotoxicity.
Polymorphisms at destinations 136, 154, and 171 are related to shifting helplessness to ovine scrapie. (These ovine locales compare to human destinations 133, 151, and 168.) Polymorphisms of thyroid profile the PrP-VRQ shape and PrP-ARQ shape are related to increased vulnerability, though PrP-ARR is related to resistance.
The National Scrapie Arrange of the UK points to breeding out these scrapie polymorphisms by expanding the recurrence of the safe allele. However, PrP-ARR polymorphisms are helpless to atypical scrapie, so this may demonstrate unfruitfulness.
Function of PRNP
Nervous system
The solid affiliation to neurodegenerative illnesses raises numerous questions about the work of PrP within the brain. A common approach is utilizing PrP-knockout and transgenic mice to explore insufficiencies and differences. Initial endeavors delivered two strains of PrP-null mice that appear to have no physiological or formative contrasts when subjected to a cluster of tests.
Be that as it may, more later strains have appeared noteworthy cognitive variations from the norm. As the invalid mice age, a stamped loss of Purkinje cells within the cerebellum results in diminished engine coordination.
Be that as it may, this impact isn't a coordinated result of PrP's nonattendance, and or maybe emerges from expanded Doppel quality expression. Other watched contrasts incorporate decreased stretch reaction and expanded investigation of novel situations.
Circadian cadence is modified in invalid mice. Fatal familial sleep deprivation is thought to be the result of a point transformation in PRNP at codon 178, which authenticates PrP's association with sleep-wake cycles. In expansion, the circadian direction has been illustrated in PrP mRNA, which cycles routinely with day-night.
Memory
Whereas invalid mice show typical learning capacity and short-term memory, long-term memory combination shortages have been illustrated.
As with ataxia, usually inferable to Doppel quality expression. In any case, spatial learning, a transcendent hippocampal function, is diminished within the invalid mice and can be recuperated with the re-establishment of PrP in neurons; cect chest this shows that the misfortune of PrP work is the cause. The interaction of hippocampal PrP with laminin (LN) is urgent in memory preparation and is likely balanced by the kinases PKA and ERK1/2.
Encourage bolster for PrP's part in memory arrangement is inferred from a few populace thinks about. A test of solid youthful people appeared to expand long-term memory capacity related to an MM or MV genotype when compared to VV. Down disorder patients with a single valine substitution have been connected to prior cognitive decline.
Several polymorphisms in PRNP have been connected with cognitive impedance within the elderly as well as earlier cognitive decline. All of these things explored contrasts in codon 129, demonstrating its significance within the general usefulness of PrP, in specific concerning memory.
Neurons and synapses
PrP is shown in both the pre-and post-synaptic compartments, with the most prominent concentration within the pre-synaptic parcel. Considering this and PrP's suite of behavioral impacts, the cect whole abdomen neural cell capacities and intuition are of specific intrigue. Based on the copper ligand, one proposed work casts PrP as a copper buffer for the synaptic cleft.
In this part, the protein seems to serve as either a copper homeostasis component, a calcium modulator, or a sensor for copper or oxidative stress. Loss of PrP work has been connected to long-term potentiation (LTP). This impact can be positive or negative and is due to changes in neuronal excitability and synaptic transmission within the hippocampus.
A few investigations show PrP inclusion in neuronal advancement, separation, and neurite outgrowth. The PrP-activated flag transduction pathway is related to the axon and dendritic outgrowth with an arrangement of kinases.
Immune system
Even though most consideration is centered on PrP's nearness within the nervous framework, it is additionally plenteous in safe framework tissue. PrP-resistant cells incorporate hematopoietic stem cells, and develop cect pelvis lymphoid and myeloid compartments and certain lymphocytes; moreover, it has been identified in common executioner cells, platelets, and monocytes. T cell actuation is gone by a solid up-regulation of PrP, even though it isn't imperative.
The need for immune response to transmissible spongiform encephalopathies (TSE), neurodegenerative infections caused by prions, may stem from the resistance to PrPSc.
Muscles, liver, and pituitary
PrP null mice provide evidence for a role in muscle physiology when subjected to a forced swim test that revealed decreased locomotor activity. Aged mice overexpressing PRNP showed marked muscle tissue destruction.
Although present, very low levels of her PrP are present in the liver and may be associated with liver fibrosis. Little is known about mammalian folate level pituitary PrP, although its presence in the pituitary has been shown to affect neuroendocrine function in amphibians.
What are the Diseases caused by PRNP
Changes within the PRNP quality have been related to a few uncommon, acquired neurodegenerative disarranges, collectively known as prion maladies.
These diseases are caused by the aggregation of misfolded prion protein (PrPSc) within the brain and other tissues, which leads to the dynamic annihilation of brain tissue and in the long run, passing.
Prion maladies can influence both people and creatures and incorporate:
Creutzfeldt-Jakob illness (CJD)
A quickly dynamic and deadly brain clutter that influences around 1 in 1 million individuals around the world each year.
Gerstmann-Straussler-Scheinker disorder (GSS)
An uncommon, acquired clutter that influences the anxious framework and regularly starts in mid-life.
Deadly familial sleep deprivation (FFI)
An uncommon, acquired clutter that influences the sleep-wake cycle and leads to dynamic dementia and eventually, passing.
Kuru
An uncommon and deadly brain clutter that was once found among individuals in Papua Unused Guinea who practiced shape cannibalism.
Bovine spongiform encephalopathy (BSE)
A prion malady that influences cattle and is commonly known as "frantic bovine malady."
The onset and movement of prion maladies can shift depending on the particular malady and the fundamental hereditary transformation included.
In any case, all prion illnesses are characterized by the collection of irregular PrP within the brain and other tissues, driving the characteristic neurodegeneration seen in these clutters.









