Six of the ten species that make up the Baylisascaris genus of ascaridoid nematodes are found in the New World. The majority of the Baylisascaris species have a common life cycle.
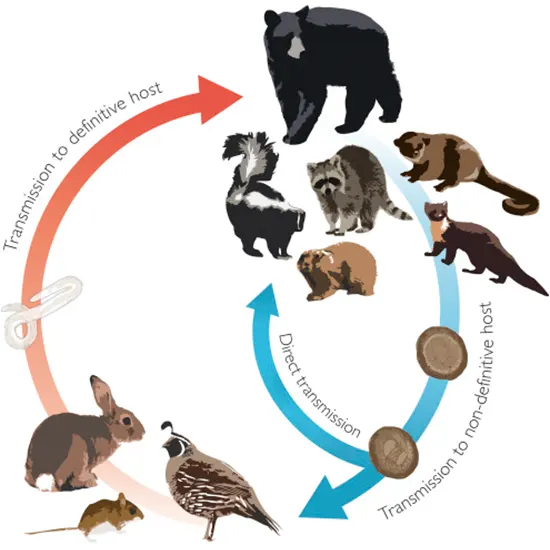
Six of the ten species that make up the Baylisascaris genus of ascaridoid nematodes are found in the New World. The majority of the Baylisascaris species have a common life cycle, with smaller prey animals acting as paretic (or intermediate) hosts and carnivorous mammals or marsupials acting as definitive hosts.
However, one rodent species is distinct because it only has one host. The raccoon roundworm, B. procyonis, is a well-known cause of severe to fatal neurologic disease in people and many other species, and it has been the subject of extensive investigation.
Genus Baylisascaris
The Ascarididae family include the genus Baylisascaris, which is primarily composed of heterogeneous nematodes with definite carnivorous hosts. The raccoon roundworm, Baylisascaris procyonis, is by far the most well-known and thoroughly researched member of the genus, Ultrasound primarily because of its link to serious neurologic disease in both humans and a wide range of animal species. As a result, compared to B. procyonis, many other Baylisascaris species are poorly understood. Here, we evaluate what is known about non-raccoon Baylisascaris spp. in the Americas today.
In 1968, the parasitologist H. A. Baylis of the British Museum of Natural History was recognised with the official description of the genus Baylisascaris, which bears his name . Some former Ascaris and Toxascaris members were combined into this genus, which was primarily distinguished from other ascarid genera by the presence of pericloacal rough patches and sub-ventral postcloacal papillae X-Ray Chest (as opposed to Toxascaris' lack of sub-dorsal postcloacal papillae) (Sprent, 1968). B. devosi, B. columnaris, B. procyonis, and B. laves, formerly belonging to the genus Ascaris, have been reallocated to Baylisascaris, while B. transfuga and B. melis once belonged to the genus Toxascaris.
In the context of zoonotic ascarids, Baylisascaris and Toxocara are frequently addressed together despite their biological similarity. However, they belong to different subfamilies and are clearly isolated from one another.
Life cycle
All members of the Baylisascaris species, with the exception of B. laevis, depend on carnivores as their sole hosts. The majority of these carnivore-infecting organisms also make use of a variety of natural paretic hosts, while some evidence suggests that these hosts may really Ultrasound Whole Abdomen be intermediate hosts (see below). The definitive host's small intestinal lumen is where adult nematodes mature and consume host digest-a. Based mostly on data from B. procyonis, females are exceptionally fecund and can release more than 100,000 eggs per worm every day, which are excreted in the faeces.
Though experimental infections demonstrate that egg inoculation can typically only establish infection in young definitive hosts, it is anticipated that definitive hosts will develop some tolerance to infection via the direct route with age.
The eggs of Baylisascaris spp., like those of other ascarids, are protected by an adhesive proteinaceous layer that gives them a high level of resistance to desiccation, freezing, heat (up to 62 C), and disinfectants. They may survive in the environment for years.
Larvae that are consumed by paratenic hosts hatch from eggs in the small intestine, pierce the intestinal wall, and then go through tissue migration after getting into the bloodstream. Every Baylisascaris species and host species exhibits a different pattern of migration and associated larva migrans symptoms. Visceral larva EEG migrans (VLM), ocular larva migrans (OLM), and neural larva migrans (NLM), the latter of which can result in severe neurologic disease with lasting sequelae and mortality, are three larva migrans syndromes that are well-described in B. procyonis.
Upon predation, migrating larvae frequently become encapsulated in the tissues of paratenic hosts and are infectious to permanent hosts. As demonstrated with Toxascaris, it is assumed that after being MRI ingested by the definitive host, L3 larvae mature in the small intestine mucosa before returning to the lumen at the L4 stage and moulting into adults. However, it is unclear whether additional somatic and/or tracheal migration occurs in all definitive hosts.
It's probable that the method of infection will affect whether subsequent migration takes place inside the final host. After egg inoculation, if the stage inside the egg is L2, migration outside of the gastrointestinal tract might be CBC necessary for progress to L3. Toxascaris Lenin has demonstrated that maturation can occur entirely in the intestinal wall and lumen without migration if infection occurs as a result of the ingestion of L3 larvae in host tissues.
Diagnostic characteristics
Grossly speaking, adults of the Baylisascaris spp. species resemble giant ascarids in many ways (long cylindrical body, off-white to brown colour, three prominent lips, tail tapered to a point), with females being larger than males. Sprent (1968) listed the following as the primary diagnostic characteristics of the genus Baylisascaris:
- Unlike Ascaris or Toxascaris, males have roughened areas prior and posterior to the cloaca; the arrangement of the post-cloacal papillae also differs from Toxascaris.
- Comprises every trait found in the Ascarididae.
- The presence of cervical ale, which may be conspicuous or diminished.
- There are doublets of dorsal and sub-ventral labial papillae.
excretory cell with nucleus in or behind commissural area, U-shaped.
- In most members, rather small, stout spicules less than 1.0 mm in length
Pre- and post-cloacal groups of male tail papillae are divided into various numbers depending on the species.
However, because several Baylisascaris spp. can infect multiple species of definitive hosts, it is not advisable to base species identification solely on these hosts.When necessary, confirmation of identifications should be made using widely available molecular methods for species identification.
New world baylisasacris species
Baylisasacris potosis
Recently, B. potosis, a brand-new Baylisascaris species, was identified in kinkajous (Potos flavus). Type specimens came from kinkajous kept in captivity that were born in the Cooperative Republic of Guyana . Although this species shares physical similarities with B. procyonis, genetic CSF examination of various gene targets, including the internal transcribed spacer (ITS) 2 region, 28S rRNA gene, and COX1 gene, led to its description as a distinct species.
Baylisascaris melis
Both North American and European badgers (Taxidea taxus and Meles meles, respectively) serve as the species' sole hosts for Baylisascaris melis. The parasite was initially identified in Belgian European badgers . Similar to B. transfuga in terms of morphology, B. melis has conspicuous alae as opposed to the vestigial alae found in other Baylisascaris species . Although the majority of the findings were for A. columnaris, there have been numerous instances of ascarids in wild North American badgers that are believed to be B. melis.
Baylisascaris Transfuga
Rudolph first described Baylisascaris transfuga in 1819 as Ascaris transfuga; it was then redescribed as a Toxascaris species in 1922 (Baylis and Danbury, 1922). In the end, Baylisascaris transfuga was named the type species for this genus by Sprent (1968), who also formally defined the Baylisascaris genus.
This species can be distinguished from other Baylisascaris spp. using morphological traits and/or molecular methods . Adult Baylisascaris transfuga can be distinguished from other species morphologically by its spicule length, which is estimated to be between 0.80 and 0.92 mm, its 46–70 precloacal papillae, its salient alae, its denticles in equilateral triangles, and its saddle-shaped median lip lobe.
Conclusion
The Baylisascaris genus has a wide range of significance for domestic animals, wild animals, and human health. However, there are a lot of knowledge gaps about species besides B. procyonis, which are mostly caused by a dearth of recent surveys and the use of molecular technologies to study the ecology of these parasites.
For these additional, "neglected" Baylisascaris spp., field investigations clarifying crucial life cycle traits are severely absent. In order to more accurately determine species diversity, species validity, host range, and disease produced by these parasites in wild definitive and paratenic hosts, these future field initiatives should ideally combine contemporary genetic methodologies with classical morphologic analysis.









