Given the tiny size of the organ, small intestinal neoplasms are uncommon. The alimentary canal, also known as the digestive system, comprises about 75% of its length and about 90% of its mucosal surface. This organ is also...
Introduction
Given the tiny size of the organ, small intestinal neoplasms are uncommon. The alimentary canal, also known as the digestive system, comprises about 75% of its length and about 90% of its mucosal surface. This organ is also referred to as the small intestine or small bowel. However, small intestinal cancers make up only 0.6% of all cancer cases in the United States and less than 5% of all gastrointestinal malignancies (GI) cases. Small intestine cancers are uncommon, but they are on the rise in the industrialised world, where the incidence is thought to have increased by over 100% over the past 40 years.
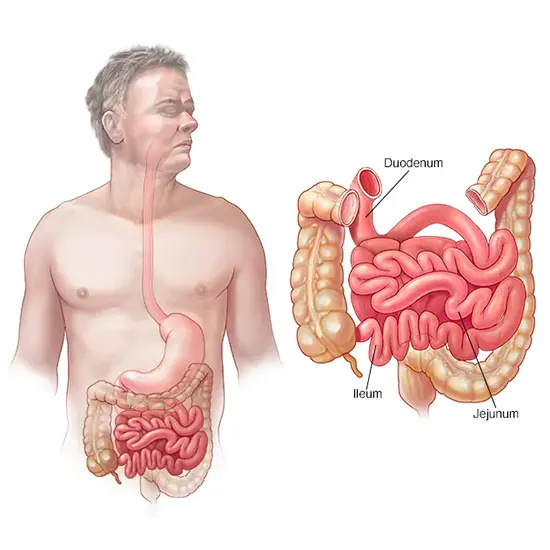
The main site of nutrient end absorption from food, including proteins, lipids, and carbohydrates, is the small intestine, which is situated between the stomach and the large intestine. The duodenum, jejunum, and ileum make up its three separate sections. Most of the digestive enzymes produced by the body are released in the duodenum, which is also, strangely enough, where small intestine tumours most frequently occur. This tiniest portion of the small intestine is home to 55-82% of all small bowel neoplasms. The ileum is responsible for the remainder 7-17% of these cancers, while the jejunum is responsible for 11-25% of them.
Small bowel adenocarcinoma (SBA), which accounts for 40% of instances, and neuroendocrine tumours, which account for another 40%, are the two main aetiologies of small intestine cancers. The remaining 20–25% are made up of lymphomas, sarcomas, and gastrointestinal stromal tumours . The rarity of SBA has made it challenging to fully characterise the pathogenesis of these two varieties; however, the stark differences in incidence indicate that SBA has a significantly different pathogenesis from colon adenocarcinomas, elicited by a different range of mutagens.
Up to 50% of SBA cases have been linked to the DNA integrity-checking tumour suppressor gene (TSG) p53, while 10% of SBA cases have been linked to the TSG adenomatous polyposis coli (APC). Another 10–40% of SBA instances have beta-catenin pathway mutations, which are critical for cellular signalling and proliferation. Over 50% of SBA instances have been linked to KRAS, an oncogene that typically aids in cellular signalling and proliferation [9,10,11]. Many, but not all, tumour suppressor and oncogenes, such as p53, KRAS, and beta-catenin, as well as predisposing mutations (like APC and KRAS), have been linked to the emergence of SBA, and recently discovered molecular therapies involving such targets have shown promise.
Etiology And Risk Factors
The growth of the mucosal epithelial cells covering the small intestine leads to the development of adenocarcinomas. Similar to the large intestine, these benign polyps can develop immortality and, after a latent time of 10–20 years, develop into adenocarcinomas. The progenitors of adenocarcinomas are adenomas, which are polyps of granular cells that secrete mucus ]. Only 10% of adenomas in the large gut develop into adenocarcinomas ]. It is unclear if the same percentage holds given the rarity of small intestine cancers and the absence of a routine screening method similar to a colonoscopy. Small bowel neoplasms make up less than 5% of all GI cancers despite making up 90% of the mucosal surface of the digestive system, suggesting a distinct difference in pathogenesis.
The shorter time spent passing through the small intestine, which reduces exposure to dietary carcinogens, and the reduced density of microbiota, which may transform bile salts into carcinogenic deoxycholic acid, are theories for this difference . The duodenum, where bile and pancreatic juices are discharged to assist in digestion, is where small intestinal adenocarcinomas are most frequently found.
By contrast, neuroendocrine tumours, also classified as carcinoid tumours, are most common in the ileum. They are defined as epithelial tumours with primarily neuroendocrine differentiation. NETs are rare and slow-growing, and while some of their features are shared among all, most of their features are unique to their organ of origins. These tumours arise from neuroendocrine cells, such as enterochromaffin cells, scattered throughout the small intestine (and the entire GI tract). As their name suggests, these cells produce hormones and cytokines to regulate digestive system function. NETs have been found to be genomically stable tumours, meaning they acquire few genetic mutations over the process of carcinogenesis
however, studies suggest that to compensate, many display dysregulation of microRNAs.
The remaining 20–25% of small intestine neoplasms are lymphomas and sarcomas, or cancers of the connective and smooth muscle surrounding the intestines.
Risk Factors That Cannot be Changed
- Ethnicity and race: African Americans in the US are 1.6–1.8 times more prone than white Americans to develop small intestine cancer. The least prone to contract the illness are Alaskans, Native Americans, Pacific Islanders, and Asians. The mortality rate among African Americans is also greater than average.
- Age: In the United States, 66 is the typical age at which small intestine cancer is diagnosed. Although the median age of diagnosis is similar between the United States and the United Kingdom, the most prevalent age for occurrence there was between 80 and 84 [17]. While the "peak" age for mortality in the United Kingdom is 85-89, over a decade older than the normal life expectancy [17], the median age for small intestine mortality in the United States is 72 [2]. Small intestine cancer risk starts to rise after the age of 40, and it does not seem to balance off until the age of 90.
- Sex: Males are more prone than females to be diagnosed with and pass away from small intestine cancer. Men have poorer survival rates than women in the United States, where there is a gender disparity in incidence and mortality of about 1.3:1 and 1.6:1, respectively [2]. Interestingly, survival rates for men are better than those for women in the United Kingdom, despite the fact that men are still more likely to be diagnosed. The sex difference in small intestine bowel incidence and mortality may be caused by variations in nutrition, exposure to carcinogens, and metabolic rate, among other factors.
- Heritable alterations: A germ-line APC gene that predisposes individuals with familial adenomatous polyposis (FAP) to the development of adenoma polyps. By the time they are 10 to 12 years old, FAP patients have thousands of polyps developing in their intestinal lining. By the age of 40, almost all FAP patients will have been told they have colorectal cancer due to the exponentially increasing chance that these adenomas will develop into adenocarcinomas. The second most typical location for cancer in people with FAP is the small intestine. About 5% of 1255 patients in research had been identified as having small bowel adenocarcinoma. (SBA). The duodenum hosted fifty percent of those tumours.
Changeable Risk Factors
-
Diet: With correlation coefficients of 0.61 and 0.75, respectively, diets rich in animal fat and protein have been linked to a greater risk of small intestine cancer . A different case-control study discovered that people who consume a lot of red and salt-cured/smoked meat have a 2-3 fold higher chance of developing cancer . Males (but not females) who consumed large amounts of heterocyclic aromatic amines, which are present in smoked and fried meats, were found to have an increased chance of developing cancer in another case-control research . While a different research disputed the idea that meat causes cancer in particular, it did discover a link between small intestine cancer and saturated fats, which are prevalent in meats.
- Alcohol: While other studies have not, some have identified a weakly positive correlation between alcohol use and SBA. One European research found that consumption of beer and spirits increased the incidence of SBA while red wine consumption did not . Many people assume that heavy drinking has a similar effect on SBA even though small sample sizes due to the rarity of the disease prevent conclusive findings. Heavy drinking had been found to increase the risk of CRC by 20–40%, leading many to believe that it does.
- Smoking: While one European study only found a significant impact on NET several studies have found that smoking raises the risk of both SBA and NET. Like with alcohol, smoking has a more definite impact on CRC, and with a relative risk of, the IARC has come to the conclusive conclusion that tobacco use causes CRC.
- Obesity: With varying degrees of success, numerous studies have looked into the relationship between fat and small intestine cancer. According to one research, people with obesity have a relative risk of 2.8 [65]. Another strange study put the relative chance for overweight people at 1.44 and for obese people at 1.1. Similar findings from other studies were reported as an increased risk among obese men but not obese women or an increased risk among obese white veterans but not obese black veterans. Body mass index (BMI) had no impact on small intestine cancer, or even had an inverse effect, according to several studies.
- Biliary Tract Conditions: Gallstone sufferers have a higher chance of developing small intestine cancer, according to a Danish study. The majority of the tumours were NET-type cancers. It has been demonstrated that consuming a diet high in fibre and avoiding obesity lower one's chance of developing gallstones. Another study supported the results but came to the conclusion that the rise in NET diagnoses may be related to improved medical surveillance for gallstones. A competing theory suggests that people with gallstones frequently produce more bile than usual and that bile can in fact be carcinogenic.
- Vitamins and minerals: One study which intended to uncover an association between ultraviolet-B radiation and small intestine cancer, in fact, contradicted its own hypothesis, finding those at northern latitudes were most at risk, perhaps due to vitamin D deficiency. Interestingly, vitamin D has in fact been shown to reduce CRC However, long-term consumption of certain medications, such as non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids, which seem to lower the risk of CRC, have not been found to have the same effect on small intestine cancer
Preventive Measures
Small intestine neoplasm incidence has been steadily rising in the developed world, which indicates that environmental factors may be contributing to the disease's onset. Only 20% of small intestine tumours have been linked to hereditary or predisposing factors. In the meantime, numerous studies have linked small intestine cancer to factors like diet, alcohol consumption, smoking, obesity, and workplace risks. Studies of CRC, a close relative of SBAs, despite the paucity of study, point to notable improvements in cancer reduction among those who practise prevention via behavioural modification.
Physical activity, weight loss, smoking, and alcohol cessation, avoidance of red, smoked and processed meats, and consumption of fibres have all been shown to reduce small intestine cancer risk to varying degrees, as well as reduce the much more common CRC significantly. Avoidance of occupation-related mutagens and radiation is also likely to decrease one’s risk of numerous cancers, including that of the small intestine. While vitamin D and medications like steroids and NSAIDs have not been found to have a definitive effect on small intestine cancers, they do seem to have a protective role against CRC. Small sample sizes constrain studies into the risk factors and prevention of small intestine cancer and thus may overlook potentially preventive agents and behaviours due to a lack of statistical significance.
Summary
Although SBA is a rare neoplasm, its incidence has more than doubled in the industrialised world over the past few decades. Fortunately, increased prevention and treatment measures as well as an increase in the proportion of neuroendocrine tumours (which have a better prognosis compared to adenocarcinomas) have prevented mortality from keeping up with incidence.
Better monitoring and diagnostics may have contributed to the rise in incidence, but other factors, such as the rise in obesity, smoking, alcohol consumption, and red and processed meat consumption, may also have played a role.
The underlying hereditary or predisposing conditions in about 20% of instances are most likely to blame. Adenocarcinoma survival rates are still low, but behavioural change could avoid thousands of deaths annually.
















