A growing issue, BK virus nephritis poses a risk to enhancing renal transplant graft survival. It is yet unknown how this illness came to be. Declining acute rejection rates and the use of potent immunosuppressive drugs have...
A growing issue, BK virus nephritis poses a risk to enhancing renal transplant graft survival. It is yet unknown how this illness came to be. Declining acute rejection rates and the use of potent immunosuppressive drugs have been associated with an increase in BK virus infection prevalence in recent years.
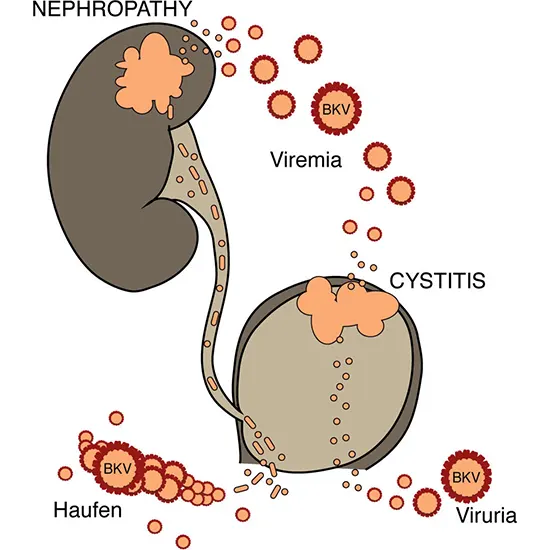
Patients with this infection typically have nephritis and/or viremia without or with decreasing renal function. Based on the presence of the virus or its effects in the urine, blood, or renal tissue, this condition can be diagnosed. Between 30 and 60 percent of individuals with BK virus nephritis experienced transplant failure in the past. In recent years, improved outcomes have been achieved by combining early detection, fast diagnosis, and interventions that include preventive measures.
The patient's initials, in whose body it was first discovered in 1971, gave rise to the moniker "BK" . Researchers from the University of Pittsburgh released their findings regarding the following observed case in 1995 . Since Biopsy then, BK virus (BKV) infection and BKV nephritis (BKVN) in renal transplant recipients have been extensively documented.
Its pathophysiology and contributing factors to its recent increase in prevalence are still poorly understood. The rising prevalence of this disease in recent years may be due to increased knowledge, improved physician recognition of this infection, and the accessibility of better diagnostic technologies.
A year after transplantation, the prevalence of BK viremia is over 20% , which is greater than the 13% reported for 2003 for the prevalence of acute rejection. In relation to BKV infection, this review examines Urine Routine the pathophysiology, clinical characteristics, management, and short- and long-term renal graft survival.
Pathogenesis of BKV Infection
- Host T cells' inadequate immunological surveillance
- The absence of prior humoral immunity to BKV,
- The virus's variable molecular sequence, and
- Alloimmune activation may contribute to the pathogenesis of BKVN. These specifics are covered in more depth below and have been evaluated elsewhere .
Over the past few years, cellular immunity in the emergence and eradication of BKV infection has continued to be a crucial topic of study. Patients with BKVN had less BKV-specific IFN-secreting cells than healthy Urine Culture control subjects. The authors reported that immunosuppressive medication was reduced and patient IFN-secreting lymphocyte counts increased, mirroring those of their healthy counterparts.
It has been demonstrated that the big T antigen and VP1 gene products of the viral genome both contain epitopes necessary for CD4+ and CD8+ cell recognition . Provenzano et al. shown within this body of work that CD8+ T cell responses were elevated at particular places within the major T antigen p53 binding area.
Two VP1 capsid protein epitopes that were recognised by cytotoxic T cells were demonstrated by Chen et al. When compared to kidney transplant recipients, these regions were discovered to be differently recognised in healthy people. A stronger T cell response was linked to a lower viral burden, whereas a weaker response was linked to a greater viral load and longer-lasting viral infection.
Humoral immunity may be involved in the pathogenesis of BKVN because people who have had a history of immunity to BKV may not get clinically ill, regardless of the amount of viral copies that are circulating. According to Bohl et al.'s findings recipients of kidneys from seropositive donors were more likely to acquire BK viremia than receivers of kidneys from seronegative donors. However, there was no statistically significant variation in viremia according to recipient serostatus.
In contrast, recipient seronegativity was discovered by Smith et al. to be a significant risk factor for the development of BKVN in a paediatric population (P = 0.01).
BKVN is more common in renal transplant patients than in liver and heart transplant recipients, suggesting that alloimmune activation may play a role in renal grafts with BKV activation and frank nephritis.
According to Awadalla et al. findings, alloimmune activation may have a role in the correlation between BKVN occurrence and a larger degree of HLA mismatches. Researchers at Emory University demonstrated that polyoma viral nephritis only happens in the presence of alloimmune activation using a mouse polyoma transplant model.
This explains why this is particular to renal grafts and how subclinical alloimmune activation in renal grafts may cause BKV replication and nephritis. It is intriguing that Drachenburg et al.'s research revealed a negative correlation between the number of HLA matches and patients' graft survival.
Clinical manifestations
BKVN is becoming more widely acknowledged as an early occurrence that happens during the first year following transplantation .
Patients with BKVN are typically asymptomatic, and their condition is only discovered when they develop renal insufficiency. In virtually all cases of BKVN, BKV DNA is found in the plasma and urine .
Rarely, hydronephrosis due to renal dysfunction secondary to ureteric stricture is found , and multi-organ failure due to severe systemic illness has been recorded.
Diagnosis
Patients with BKVN have been found to have decoy cells in their urine, which are made up of renal tubular cells that have been infected and had their nuclei changed by viral inclusions. Although a KFT sensitive (100%) test, the presence of decoy cells has a modest 29% positive predictive value for the diagnosis of BKVN . Generally speaking, it appears that the presence of decoy cells is a reliable screening test but not a BKVN diagnosis.
BKVN has been diagnosed by measuring the viral load in blood and urine using viral DNA or mRNA to VP-1. At least 50% of transplant recipients had urinary BKV DNA, but because testing circumstances can vary so much, it is difficult to standardise this procedure for sure identification . Urinary VP-1 mRNA load has been recommended by several authors as a way to identify viral replication that is active .
In the first year following a kidney transplant, 15 to 30% of patients have BKV DNA quantification in plasma, which is a critical diagnostic tool . PCR has a sensitivity and specificity of 100 and 88%, respectively, for USG KUB quantifying BKV DNA in plasma; however, not all patients with BK viremia develop nephritis (positive predictive value)
Treatment
Immunosuppressive therapy modifications, either with or without antiviral medications, have been the focus of treatment for BKV infection. To treat this infection, a number of immunosuppressive therapy modification regimens have been used. These include stopping an agent, cutting back on an agent, changing immunosuppressants within the same class or to a different class, and avoiding steroids.
Patients who had BKV infection and were unintentionally given an anti-lymphocyte drug or pulse corticosteroids for presumed acute rejection provided evidence for this method by having rapid progression towards transplant failure.
Viremia has been cleared successfully by stopping the use of a single immunosuppressive medication, antimetabolite (MMF or azathioprine), when it is detected.Elimination of viremia and preservation CT KUB of renal function have also been accomplished with reduction of immunosuppression by halving both antimetabolites and calcineurin inhibitors.It has been claimed that avoiding steroids will make BKV infections less common.
Prevention
Researchers are now considering a preventive strategy to treating BKV infection because it is difficult to identify the pathophysiology of this virus and because there is no safe and effective antiviral medication.
The condition can be identified by looking for BKV DNA in blood or urine, and individuals with viremia or viruria can have their immunosuppression reduced in advance. Researchers now choose to utilise viremia as a better marker for preemptive decrease in immunosuppressive medication due to the higher prevalence of viruria compared to viremia and the lack of a strong link with viruria.
Patients with BKVN at our facility now have better graft survival thanks to aggressive post-transplantation screening, early disease detection, and preemptive reduction in immunosuppressive medication.









